Pigmentation in humans derives from the amount of melanin produced by specialized cells named melanocytes.
The absence of melanin is the cause of albinism, a genetic disease produced mainly by mutations in tyrosinase the key enzyme in melanin synthesis.
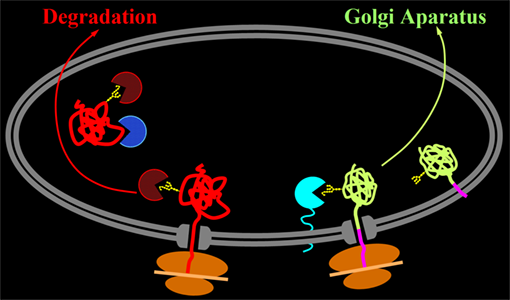
From the over one hundred mutations found in type I oculocutaneous albinism, we have investigated those affecting the anchoring of tyrosinase to the membrane. Soluble tyrosinase, lacking its transmembrane domain was retained in the endoplasmic reticulum (ER) and its trafick towards the target organelle, the melanosome, drastically impaired.
Thus, the cells expressing soluble tyrosinase remained unpigmented, indicating that tyrosinase must be tethered to the membrane in order to initiate pigmentation. To validate this hypothesis we designed tyrosinase mutants and chimeras with transmembrane domains or GPI anchors from other proteins, attached to the globular domain of tyrosinase. This approach allowed us to restore the tyrosinase activity in all cases. We were able to show also that these anchored tyrosinases were able to select chaperone calnexin for association, which helped their productive folding. By contrast, truncated tyrosinases select a different set of chaperones, calreticulin and BiP, which target the polypeptide chain to retrotranslocation and cytoplasmic degradation, instead of productive secretion.
We also found out that in the case of productive folding an additional recycling step between ER and Golgi, prior to the final release of protein for export was compulsory for the fate of pigmentation.
"Soluble tyrosinase is an endoplasmic reticulum (ER)-associated degradation substrate retained in the ER by calreticulin and BiP/GRP78 and not calnexin.",
J Biol Chem.., 280 (14), 13833-138340 (2005)
Popescu CI, Paduraru C, Dwek RA, Petrescu SM.
"Productive folding of tyrosinase ectodomain is controlled by the transmembrane anchor",
J Biol Chem., 281 (31), 21682-21689 (2006)
Popescu CI, Mares A, Zdrentu L, Zitzmann N, Dwek RA, Petrescu SM